Disperse
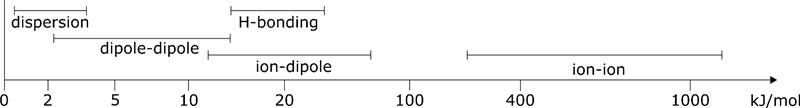
The surface energy/tension of a substance can be explained different types of interaction. Dispersive and polar interactions are differentiated. The surface tension/energy of a substance is made u pof dispersive and polar parts. Disperse interactions are weak and short-range forces between uncharged molecules. Although they are only weak, without them no liquids or solids of non-polar compounds would exist. Disperse interactions therefore have a significant influence on the behaviour of matter.
The interactions are electrostatic in nature. The electron distribution of atoms and molecules fluctuates with time. This results in short-term random charge shifts, which in turn induce dipoles.

Although these interactions are short-range and relatively weak, they make up a large proportion of intermolecular interactions. In particular, the stability of many non-polar materials, including plastics, is based on these interactions.
Since there is a strong distance dependence of these forces, it is important that the binding partners are close to each other. This is an effect that is achieved by plasma treatment. Fine cleaning removes impurities that act as release agents and the bare surface can form bonds. In addition, polar functional groups are introduced which, together with the disperse forces, contribute to the molecular interactions and produce better adhesion.
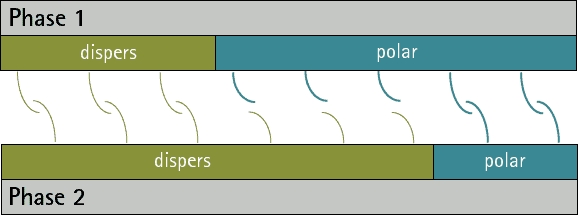
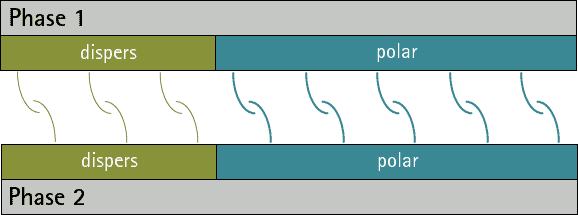
There are no substances with only polar interactions because dispersive interactions occur between all atoms and molecules. But there are substances which don’t have any polar groups. Therefore the surface tension/energy is purely dispersive.
The comparison oft he ratio between dispersive and polar parts oft he surface energy/tension fort wo phases allows a prediction oft he adhesion. The closer the ratios match the more interactions are possible between the phases. Therefore the adhesion ist o be expected higher (see figure 1 and 2). A small interfacial energy/tension shows a high potential for interaction between two phases.
Learn about our products >>



