Trade show IDS – Handheld plasma device for the dental lab and plasma components for system integration
Relyon plasma from Regensburg, a subsidiary of TDK Electronics, is presenting the piezobrush® PZ3 handheld plasma device for use in dental laboratories at IDS – International Dental Show. Moreover, the innovative implaPrep concept for demonstrating integration components for plasma activation of dental implants is introduced live.

Regensburg/Cologne. After being cancelled in spring 2021, the world’s leading trade fair for the dental community, IDS, will open its doors in Cologne from September 22-25, 2021, to present innovations and market trends to the professional audience. Relyon plasma will exhibit in hall 11.1 at booth H 48 J49 to present the benefits of plasma in the dental laboratory and implantology. On the one hand, the focus will be on the plasma handheld piezobrush® PZ3, which is primarily used in the dental laboratory for preparing prosthetics prior to bonding and color individualization. On the other hand, the focus of the trade show booth is on the implaPrep concept, which enables the plasma treatment of implants directly before implantation in order to obtain superhydrophilic implants and thus create the best possible conditions for rapid osseointegration.
Handheld cold plasma device piezobrush® PZ3 in the dental laboratory
Plasma technology has already been in use in dental laboratories for years as low-pressure plasma – however, these systems are usually very complex and expensive. With the introduction of the plasma handheld piezobrush® PZ3 last year, relyon plasma has succeeded in establishing an equally highly efficient, user-friendly plasma treatment in a compact handheld format and for a fraction of the cost.
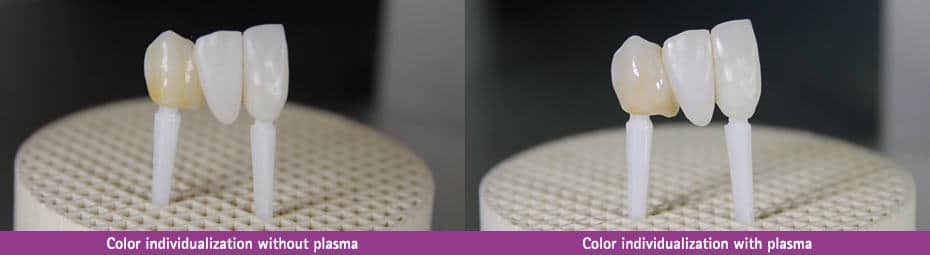
The application focus here is on the treatment of prosthetics and veneers prior to color individualization. Due to the increased surface energy, the ceramics stain, for example, is dispersed much better on the surface, which leads to an even and aesthetically attractive result. In addition, more stain can be applied within one working step; this leads to a reduction in the number of firings required and thus also shortens the processing time.
Another application is the bonding of high-performance plastics such as PEEK with composite or PMMA. In addition to the surface activation, the surface is also cleaned of organic impurities at the same time, which improves the distribution of the adhesive and enables it to bond immediately with the correct bonding partners. This results in much more stable bonds between the bonding partners, such as the prosthetic and abutment, which ultimately increases the service life of the bond and thus patient/client satisfaction.
Plasma activation of implants with the implaPrep concept
Currently, the implaPrep concept is a prototype setup that allows titanium implants to be lifted from a hydrophobic to a superhydrophilic state by a 50-second plasma treatment. This property is based on structurally neutral plasma fine cleaning and electrochemical excitation by the plasma, thus creating the basis for optimized biocompatibility and acceptance by the surrounding living tissue. The underlying increased surface energy improves the initial attachment of osteoblasts, which subsequently leads to increased bone regeneration after implantation.
This process was scientifically investigated and validated during the development phase of the plasma driver and the plasma reactor integrated in the implaPrep concept. Now it is time to collaborate with a partner from the dental industry to integrate these components developed in accordance with ISO 13485 into an existing system or to jointly establish a stand-alone device for the plasma activation of implants. Within the framework of the IDS, relyon plasma is looking for a development partner in order to transfer the device from prototype status to a series device. From now on, the focus will be on system integration, medical device approval and the subsequent establishment of an international sales network.
During a personal visit at the booth, both devices can be tested directly on site with own materials, implants and applications to get a hands-on impression of the plasma technology.
Download the press release here.